Joint Applied Medicinal Radiochemistry Facility
Team
- Univ.-Prof. PD Mag. pharm. Dr. Markus Mitterhauser (Koordinator / coordinator)
- Ap. Prof. Mag.a pharm. Dr.in Cécile Philippe (Stellvertretende Koordinatorin / deputy)
- Mag.a pharm. Dr.in Theresa Balber
- Rana Abdelaziz, MSc
- Lenka Martinak, MSc
The role of Joint AMR Facility
The Joint Applied Medicinal Radiochemistry Facility is a collaboration between the the Medical University of Vienna and the University of Vienna in the field of developing innovative radiopharmaceuticals. Radioactive labelled compounds (radiotracers) are used for diagnostic molecular imaging by positron emission tomography (PET) and single photon emission computed tomography (SPECT) as well as for therapeutic applications in nuclear medicine, particularly in the field of oncology. The Facility covers the entire spectrum of state-of-the-art radiopharmaceutical development, ranging from basic chemical and biological research and radiochemistry to preclinical research and translation into the clinic. This provides academic and industry partners a platform that comprehensively supports the development of new radiopharmaceuticals to the benefit of patients.
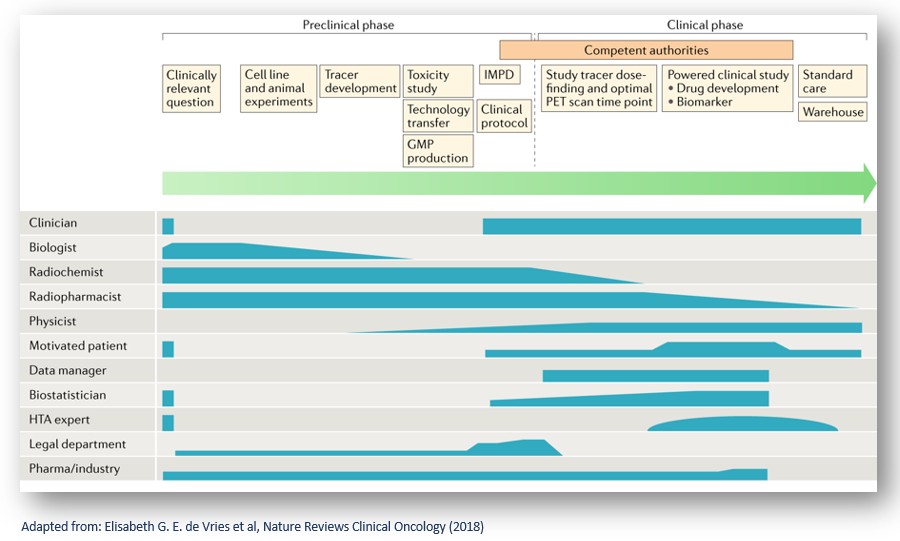
Drug development is a complex process that involves several stages and encompasses a range of methodologies. A plethora of stakeholders with diverse expertise and experience contribute to the radiopharmaceutical drug development pipeline starting from conception and target identification until translation to the final clinical setting.
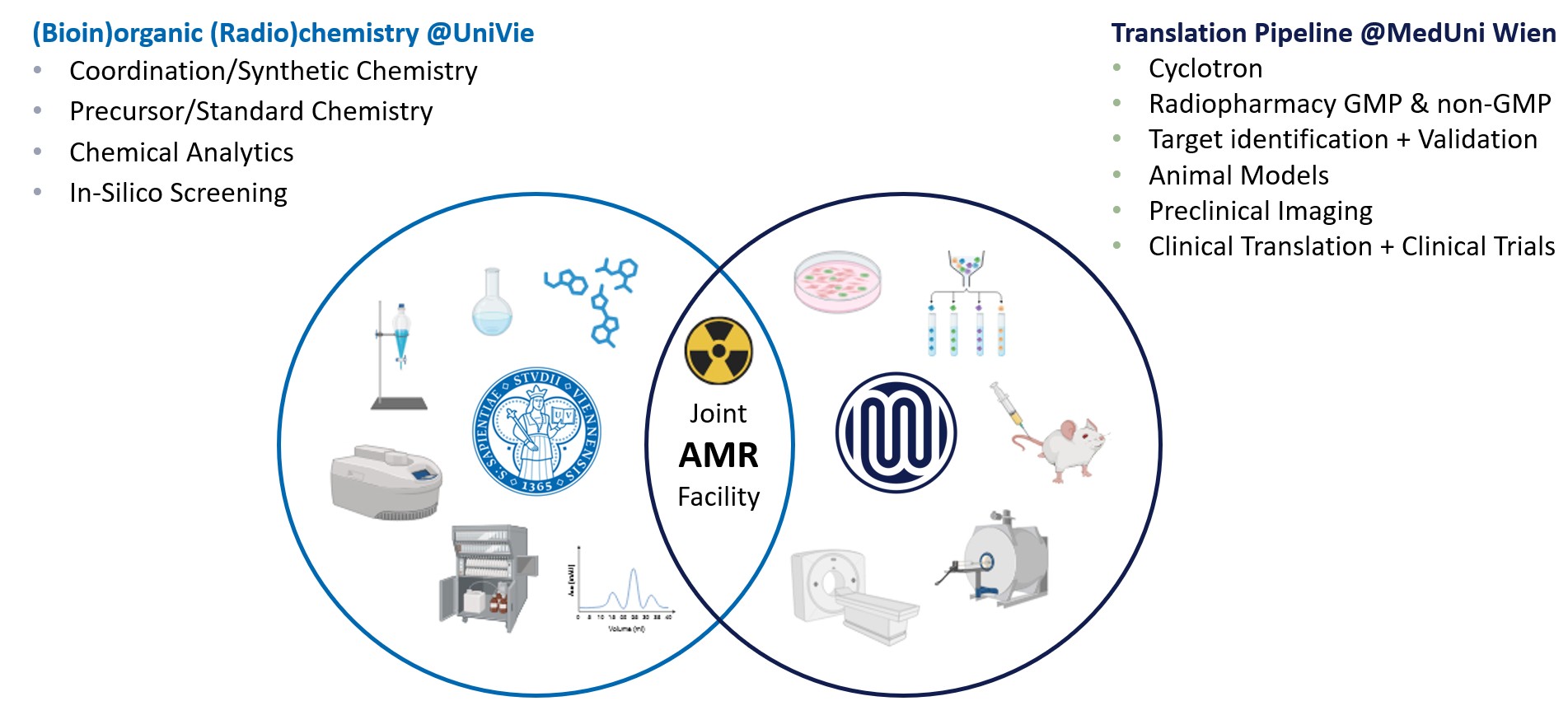
Collaboration between Medical University of Vienna and University of Vienna: Joint Applied Medicinal Radiochemistry Facility represents a strategic collaboration between the two institutions, combining their expertise and resources to enhance innovation.
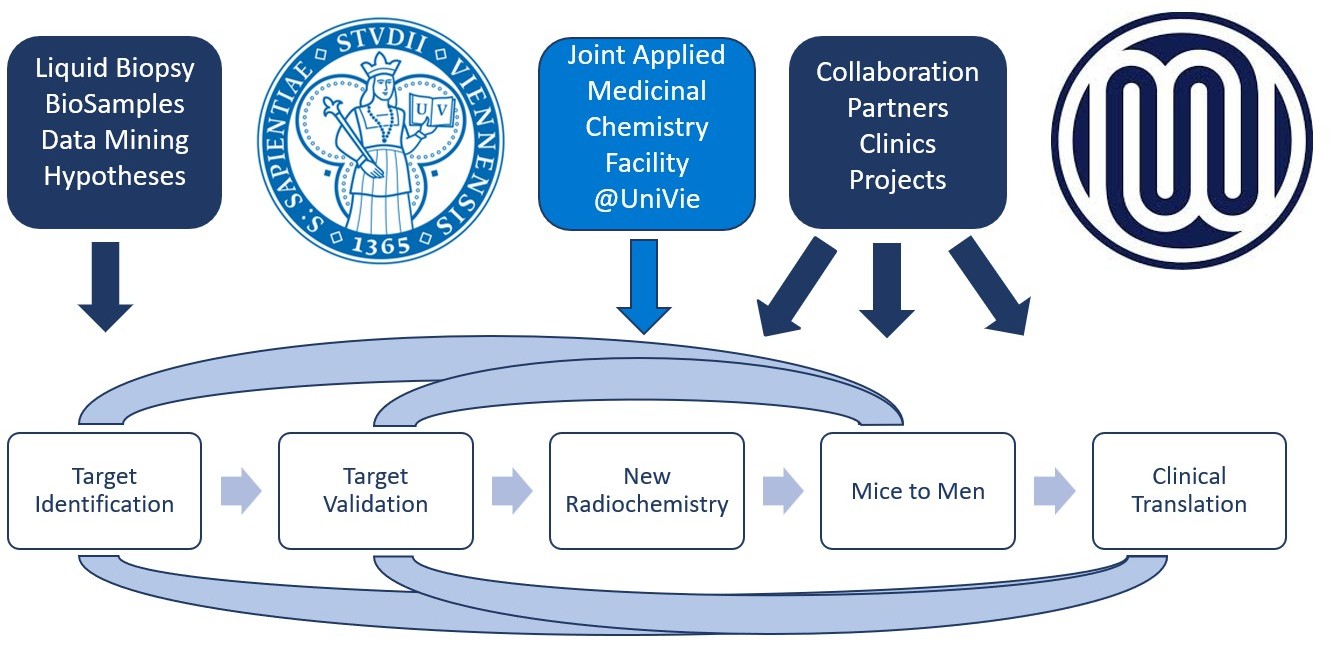
The drug development pipeline includes key steps such as target identification, validation, preclinical testing and clinical trials. Each institution has its specific role in the process.
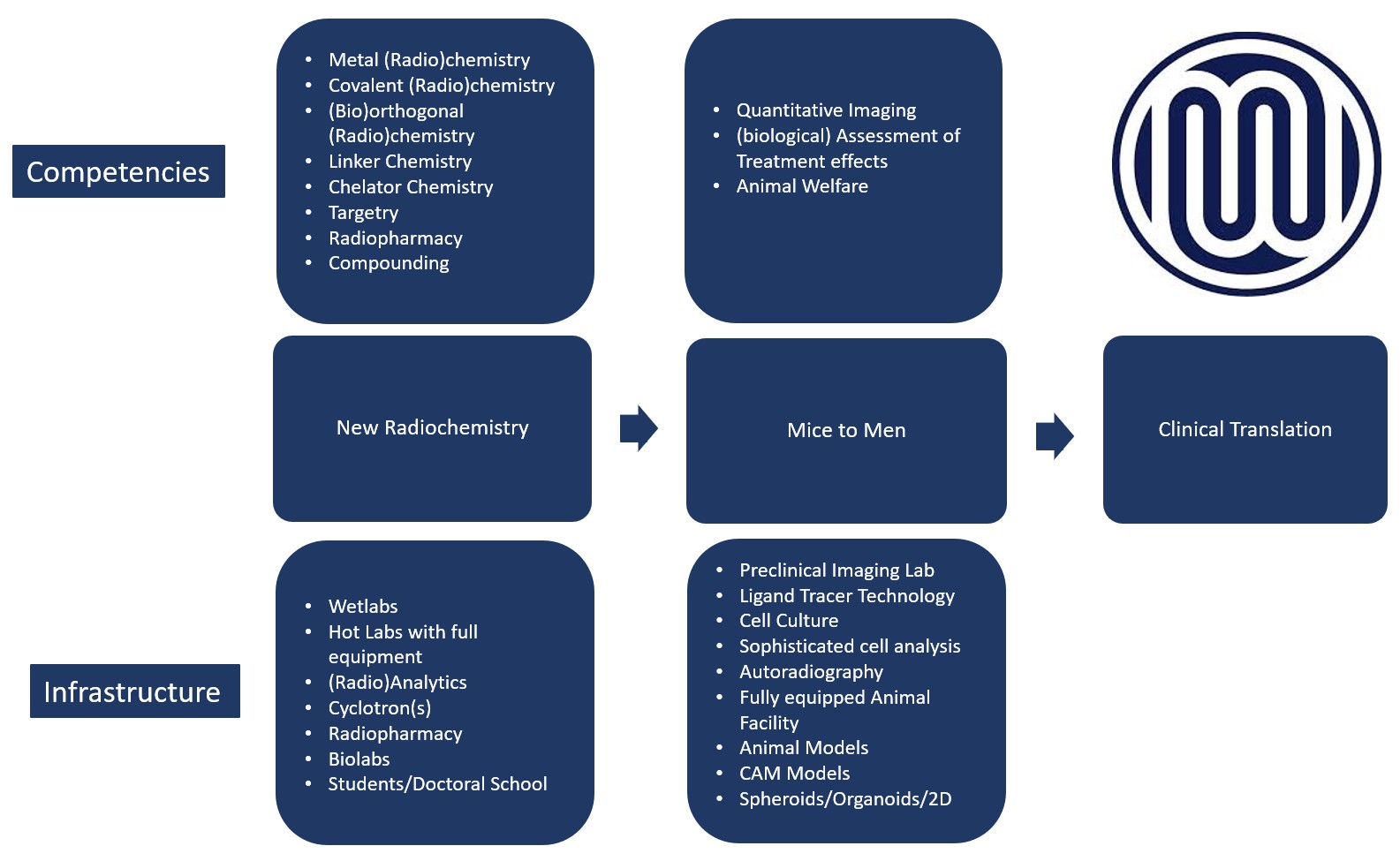
Joint Applied Medicinal Chemistry Facility research focuses on the following drug development stages: New Radiochemistry, Preclinical trials (Mice to Men) and Clinical Translation.
Publications
Giammei C. et al. (2025). Towards Dual-Tracer SPECT for Prostate Cancer Imaging Using [99mTc]Tc-PSMA-I&S and [111In]In-RM2. Pharmaceuticals, 18(7), 1002. https://doi.org/10.3390/ph18071002
Kronberger J. et al. (2025). Site-Selectively Functionalized Albumin with DFO*Maleimide for 89Zr-Radiolabeling Yields a Metabolically Stable PET Probe that Enables Late Time-Point Tumor Imaging in Mice. Journal of Medicinal Chemistry, 58(10), 4130-4142. https://doi.org/10.1021/acs.jmedchem.5c00803
Happl B. et al. (2024). Synthesis and preclinical evaluation of BOLD-100 radiolabeled with ruthenium-97 and ruthenium-103. Dalton Transactions, 53(12), 3562-3575. https://doi.org/10.1039/d4dt00118d
Happl B. et.al. (2023). Synthesis and Preclinical Evaluation of Radiolabeled [103Ru]BOLD-100. Pharmaceutics, 15(11), 2626. https://doi.org/10.3390/pharmaceutics15112626
Schmitl S. et.al. (2023). Quality Assurance Investigations and Impurity Characterization during Upscaling of [177Lu]Lu-PSMAI&T. Molecules, 28(23), 7696. https://doi.org/10.3390/molecules28237696
