Aortic aneurysms
An aortic aneurysm is a circumscribed widening of the aorta, either in the thorac, i.e., thoracic aneurysm, or in the abdomen, i.e., abdominal aorta. The majority of aneurysms in the lower half of the aorta extend to the renal arteries, i.e., infrarenal aneurysm, with or without involvement of the pelvic arteries.
The cause of aortic aneurysms is most often severe atherosclerosis, i.e., arterial calcification, which involves a multifactorial process. Chronic inflammatory changes cause destruction of the connective tissue fibers and smooth muscle cells causing weakening of the vessel wall. Risk factors for developing arteriosclerosis include nicotine abuse, hypertension, hyperlipidemia and obesity, but also age, male gender and family tendency play a role.
Distribution of aneurysms
Abdominal aorta 55%
Thoracic aorta 25%
Pelvic arteries 3%
Other arteries 27%
Abdominal Aortic aneurysms
Regarding abdominal aneurysms, 90% involve the infrarenal aorta while 10% to 30% affect the pelvic arteries.
In men, the incidence begins to rise steeply at 55, reaching a peak of 5,9% by 80. In women, the incidence rises rapidly at 70, achieving a maximum of 4,5% by 90. Overall, 5.4–6.6% of people over the age of 60 have an aneurysm.
An abdominal aneurysm is defined as an infrarenal aoritc diameter exceeding 3 cm. On average, aneurysms grow 2mm per year. However, the propensity to grow can’t be predicted. Although 15–20% of aneurysms remain stable, sudden growth can occur after a long period of stability. This is why regular imaging is necessary. The size of the aneurysms influence the risk of rupture. The annual risk of rupture is 2% in aneurysms < 4 cm, and climbs to 10% for aneurysms of 6–7 cm.
An aneurysm mostly causes no symptoms and is often found on routine imaging, i.e., abdominal x-ray or ultrasound. Large growth however can produce pressure on palpation of the abdomen, the lumbar spine or chest. Expeditious treatment is warranted for symptomatic aneurysms. Acute rupture without warning is more common and can cause life-threatening bleeding.
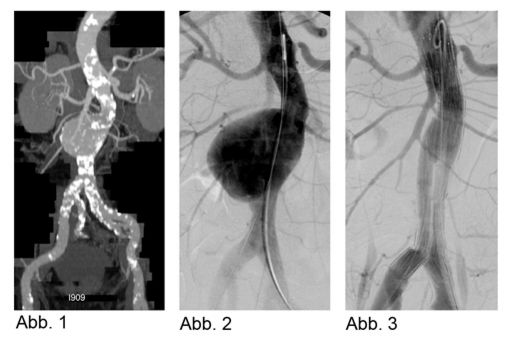
Indications for treating an aneurysm include pain, diameter ≥ 5 cm, or any aneurysm that grows > 0.5 cm per year. Abdominal sonography is the preferred modality for screening and routine follow-up of aneurysms that are well-seen. CT displays the exact size and morphology of the aneurysm (Abb. 1); MRI is done only in exceptional cases.
Alternatives to surgery include endovascular aneurysm repair (EVAR) using fabric-covered metal mesh stents. Anatomic suitability is a precondition for EVAR, i.e., adequate distance between the pelvic arteries plus an area for anchoring the stent. The stent is implanted by interventional radiologist together with anesthesiologists and vascular surgeons. Spinal anesthesia or general sedation is used for the intervention. Access is through a short incision near to the aneurysm. First angiography is done to image the aneurysm (Abb.2). Using fluoroscopy, the stent is placed over a guide wire and anchored in the infrarenal abdominal aorta. Following the implantation, angiography is performed to document the stent’s position (Abb.3).
Thoracic Aortic Aneurysm
The incidence is 6/100000 inhabitants per year.
The ascending aorta is involved in 50%, the aortic arch in 10–15%, and the descending aorta 35–45% of cases. In 25% of patients with an atherosclerotic thoracic aneurysm there is also an abdominal aneurysm. After atherosclerosis, chronic aortic dissection is the second most common cause o aortic widening.
Treatment is indicated for any aneurysm with a diameter ≥ 6 cm, even if asymptomatic. In this appropriate area, aneurysms may cause odynophagia or hoarseness due to compression of the recurrent laryngeal nerve.
By elective surgical repair of a descending aortic aneurysm, the reported 30-day mortality rate is 5–13%. Further postoperative complications include respiratory problems, neurological deficit in up to 33%, and renal insufficiency in as many as 8%. EVAR has clearly lowered the mortality and complications rates and has great significance as an alternative method for treating descending aortic and aortic arch aneurysms.
Surgery involving the aortic arch requires revision of the aortic arch branch vessels, in order to anchor the stent graft.
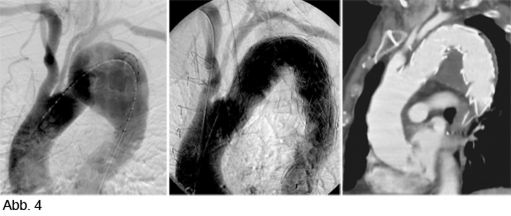
Abb 4a shows an aneurysm extending into the aortic arch and the left carotid artery, and near to the left subclavian artery origin. A stent can be performed after transposition of the carotid and subclavian arteries (Abb. 4). Follow-up CT confirms the correct position of the stent and resolution of the aneurysm.
Therapy and Follow-up
CT angiography is routinely done a few days after stent placement and annually in uncomplicated repairs.
Cardiac-CT
Cardiac-CT
Until the new millenium, the depiction of the coronary arteries was essentially done invasively, through catheter placement in the arteries, i.e., angiography (CTA). In the last few years, technical developments in computer tomography (CT) now allows us to image the coronary arteries noninvasively, by using a contrast agent. Despite the tiny diameter of the cononary arteries, tortuous course, and motion during the cardiac cycle, depicting the coronary arteries is no longer problematic. In case of doubt regarding the indication, discussion with the referring doctor will be essential.
The following paragraphs will briefly describe the technique, indications, limitations and risks of cardiac-CT.
Technique
Technical Assumptions
Both the spatial and temporal resolution have been improved with the multi-slice CT scanner. The existing three generation-old CT-Scanner achieved maximum spatial and temporal resolution though neither the x-ray source nor the 64 detectors could rotate. In one rotation, 64 slices rather than a single slice were acquired.
In order to improve the temporal resolution, it was decided that the heart rate should be 65 beats/minute. Therefore, before the exam, you may be given a ß-blocker, e.g., Beloc, to decrease your heart rate.
How the test is performed
The exam is done with you in the supine position. EKG leads will be placed, and venous access obtained. You will be told to breathe, and then hold your breath, repeatedly throughout the exam.
Ca-Scoring
A low-dose CT scan with standardized parameters will be done without contrast agent. Based upon the thickness of the calcium, the calcium content in your coronary arteries will be scored, i.e., the Agatston Score, and divided into 4 categories (0–10; 10–100; 100–400; > 400).
CTA
Once the Ca-scoring is complete, the CT Angiography (CTA) of the coronary arteries will be performed after a quick injection of ca. 90 ml nonionic contrast agent. This is the relatively radiation-intensive part of the test, i.e., 6–10 mSv. This is why precise indications are stated (see below). With our latest-generation machine, the average radiation dose for the CTA has been reduced to ca. 1mSv.
EKG-Triggering
The exam will be EKG-triggered to stop the heat motion. This is an intentional slower and more regular heartbeat. Administering the ß-blocker will keep the heart rate reduced. In those with arrhthymias, the image quality can deteriorate.
Current Indications
Proof of/ Rule out CAD
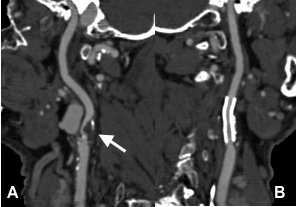
Currently, rule out CAD is the most secure and reasonable indication for A CT- Angiography (CTA) of the coronary arteries. By clear signs of acute ischemia (acute coronary syndrome) an invasive exam is immediately indicated. It is not worth wasting time with CTA. More often the strength of noninvasive CTA is to confirm CAD in patients with low pretest probability. CTA should also be done short- or medium-term in patients with no or few known risk factors but who have vague complaints, e.g., atypical chest pain. Such a patient profits to the maximum if CAD is ruled out or confirmed.
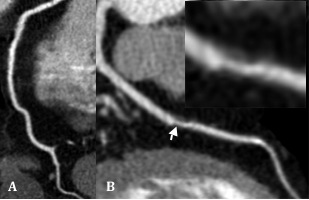
Figure: During the RCA (A) we have completely unremarkable images, The middle third of the LAD (B) has a noncalcified (arrow and Insert).
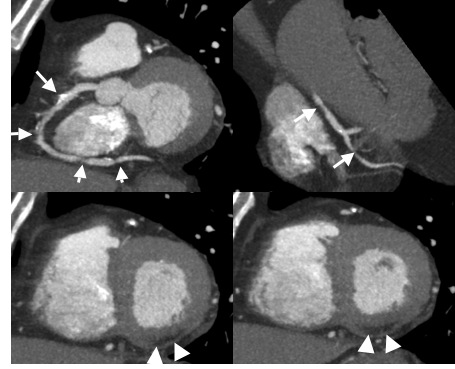
Figure: CAD proven by contrast-enhanced CTA oft he coronary arteries
72-yr-old man, whose EKG showed a non-recent MI (posterior wall).
Referred for CTA for therapy planning for proven CAD.
Several images of a relevant stenosis in a partially calcified lesion in the RCA (arrow). Consistent with the EKG report, there is an RCA lesion and a broad scar in the posterior wall.
Follow-up after bypass operation
In principal, post-operative bypass patients were the first to undergo CT-Angiography (CTA) of the coronary arteries. The non-anatomic course of the bypass and the low incidence of motion artifacts quickly established this indication in the routine.
For these patients, the noninvasive CTA is an alternative to cardiac catheterization. This is especially important because the non-anatomic course of the vessels in bypass patients can make it hard to direct the heart catheter; multiple aortocoronary bypasses; unsuccessful probe in all bypasses; LIMA/RIMA bypasses. CTA is an essential alternative.
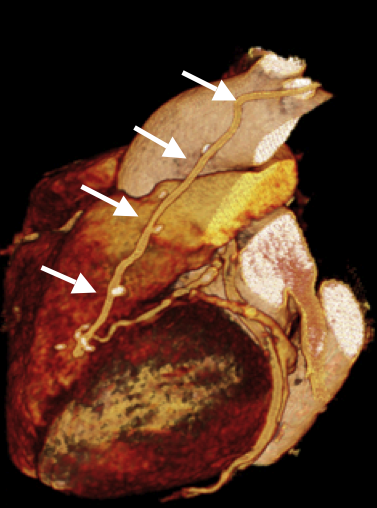
The images (Fig.) show a CT-Angiography of the coronary arteries in a 77-yr-old woman who ended up in this condition after a LIMA-and-LAD-bypasses a year ago. She complained of new angina pectoris.
The bypass appears normal (arrow), The target vessel (distal LAD) appears fragile.
Follow-up after endovascular therapy
Today, stents are found in the vast majority of coronary arteries treated by endovascular therapy. The thickness of the metal stents can interfere with judging a stent‘s integrity. Depending on the stent length, stent diameter and cross-section images of the artery containing the stent, the stent’s placement can be judged successful or not (Figure 7). Judging stent position will improve in the next years due to newer reconstruction techniques and image editing. For the time being, patients with long stents or multiple stents are not ideal for cardiac-CT.
Further Indications:
- Follow-up after heart transplantation as an alternative to yearly cardiac catheterization
- Planning electrophysiology therapy, i.e., ablation
- Rule out CAD before a big operation, i.e., clearance
- Patients with congenital cyanosis
- Patients with coronary artery anomalies
Contraindications and Limitations
Absolute and relative contraindications exist for CT-Angiography of the coronary arteries, similar to CT performed with iodinated contrast agent. The main contraindications and appropriate preparation are summarized in Table 1. To determine the possible contraindications, patients will be given a standardized questionnaire to complete before the exam (see online here)
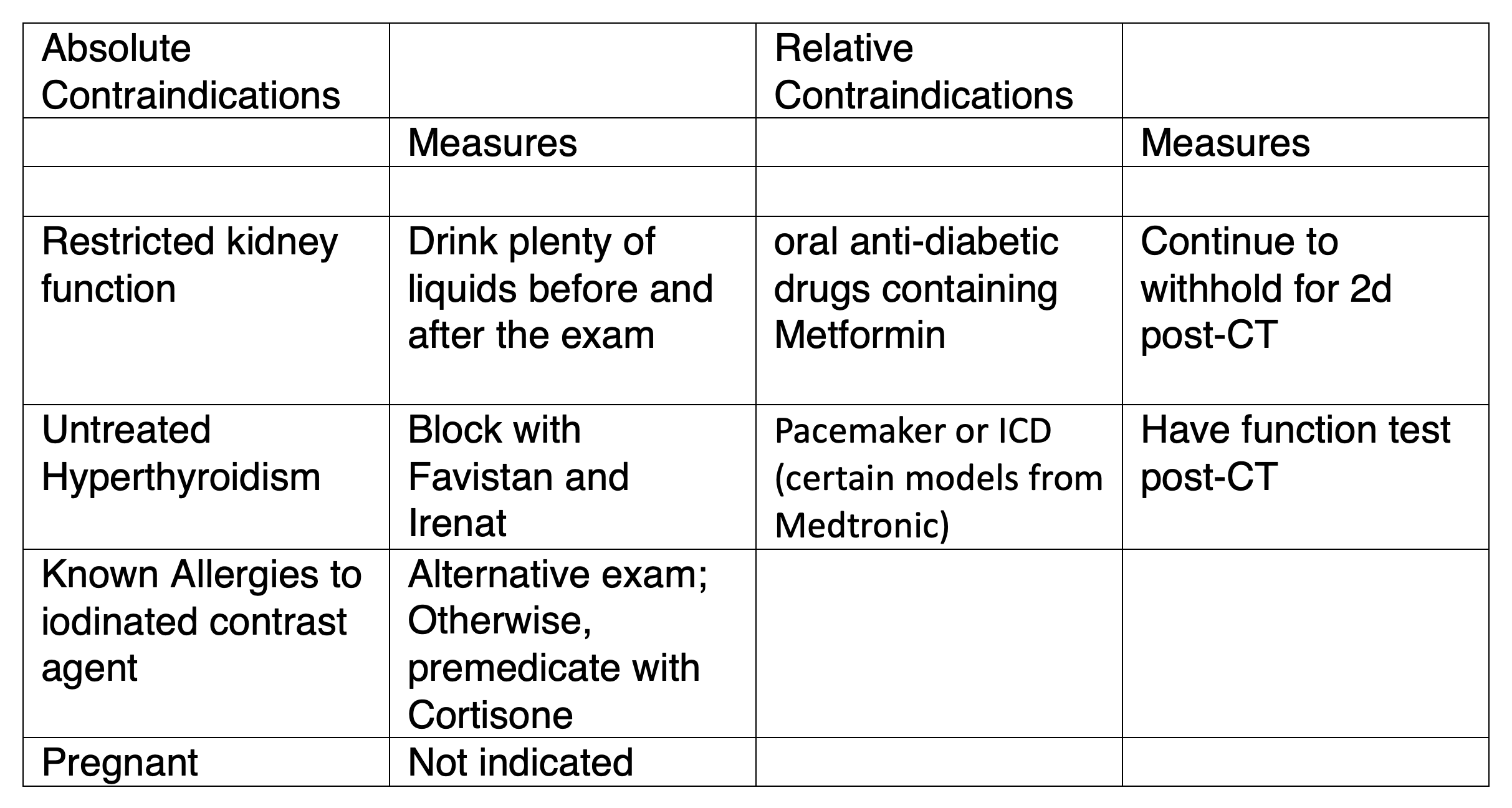
Table 1
All absolute and relative contraindications to CT contrast agent
In addition, there are further contraindications and limitations for CT-Angiography of the coronary arteries (Table 2).
Any Agatston-Score (see Ca-Scoring) that exceeds 500 causes severe beam-hardening artifacts on CT-Angiography of the coronary arteries due to the calcium. Therefore, accurate assessment of calcium is not possible. .
Despite EKG-triggering, tachycardia causes pulsation artifacts which degrade CT-Angiography image quality.
Summary
The options offered by multislice-CT-Scanner have enormously improved spatial and temporal resolution for noninvasive depiction of the coronary arteries. In particular, CTA is increasingly used to rule out CAD and for post-bypass follow-up. Due to the current limitations and risks, patient selection is restricted. Therefore, patient preparation is imperative. But with further technical developments within the next year, increased patient throughput will be possible. Since 2010, our division has had the latest-generation CT in the field, i.e., the Siemens Somatom Definition Flash. It has lowered the radiation dose for cardiac-CT. Furthermore, the quality of the exam in patients with high heart rates will clearly be better. In the future, we hope to be able to scan patients who can’t breath-hold and those who can’t lie still.
FAQ:
Can I avoid cardiac catheterization by having a cardiac-CT?
A cardiac-CT can only rule out or prove that you have CAD. For treatment, a cardiac catheterization (with stent implantation) is necessary. So if there’s a very high likelihood that you will receive treatment for CAD (i.e., definite pain, positive treadmill test, positive scintigraphy), you probably can’t avoid cardiac catheterization. The CT referral slip for cardiac-CT from your doctor should always be filled out. This will tell me if the exam is simply being done before cardiac catheterization or if he has another query.
When is a cardiac-CT indicated (meaningful)?
- With clinical pain, to rule out coronary artery disease (CAD)
- With known CAD for treatment planning
- For suspected CAD and reserved cardiac catheterization
- As a follow-up after bypass surgery
- As a follow-up after cardiac transplantation
- As preparation for planned electrophysiology treatment
Are there any contraindications for cardiac-CT?
In principle, cardiac-CT is none other than a CT that uses contrast agent. Therefore, contraindications are the same as those for contrast agents (see above, and also the information sheet homepage, www.oerg.at) , i.e., contrast agent allergy, renal insufficiency, hyperthyroidism. The only other contraindication is tachycardia, atrial fibrillation, and high-grade calcifications of the coronary arteries (which will be apparent after the first part of the exam, i.e., calcium scoring).
Should one have a cardiac-CT screening test every year?
No. At this time, it is not a suitable screening test. A cardiac-CT should only be done if your doctor considers it is necessary and meaningful.
Do you need special preparation for cardiac-CT?
No. As with all CTs where contrast agent will be injected, you must fast 4 hours.
How long does a cardiac-CT last?
Including preparation, i.e., EKG lead placement, the exams takes 15 minutes maximum
CT Angiography
Due to enormous technical developments in CT during the past decade, vessels in all parts of the body can now be depicted in high-resolution. Therefore, contrast agent is simultaneously given during CT in order to see the vessels. Due to CT’s high speed, large vessels such as the aorta, and complete leg arteries can be depicted. This allows us to see most of the changes in the arteries noninvasively. That means a direct puncture of the artery, as in angiography, is not necessary.
In principle, CT-Angiography (CTA) is the same for all areas of the body. The technique is little-altered
Technique
Technical Requirements
We can depict the vessels since multi-slice CT has made possible high spatial and temporal resolution. With a third-generation CT scanner in our division, we can obtain images with high spatial and temporal resolution. With this device, neither the beam nor the 64 detectors rotate around the patient. With each rotation you acquire 64 slices simultaneously instead of just a single slice.
Course of the examination
You will lie in the supine position for the exam. Usually, EKG leads will be placed on your chest, venous access will be obtained for contrast agent administration, and you will be asked repeatedly to breathe in and then breath-hold. .
Ca. 90–110 ml of nonionic contrast agent will be injected for the CT-Angiography.
EKG-Triggering
Exam of the thoracic aorta is performed with EKG-triggering (see below) to exclude pulsation with the heartbeat.
Below a brief explanation of the indications, limitations and risks of the exam.
Presently, these are the vascular studies our division offers:
Aorta
The principle question by CT-Angiography of the aorta (in the chest or abdomen) is whether the aorta is enlarged, i.e., a so-called aortic aneurysm. The urgency with which an aneurysm is treated will depend upon the diameter of the aorta. Treatment is warranted when an aortic aneurysm exceeds 6 cm in the chest or > 5–5.5 cm in the abdomen. When a thoracic aortic aneurysm is the concern, the exam will be done with EKG-triggering (see below) to exclude pulsation with the heartbeat.
CT-Angiography, like MR-Angiography (see below) is not only the most precise imaging method to measure the aortic aneurysm, but also the optimal way to decide if treatment is required. All parameters necessary to determine if endovascular treatment, i.e., stent implantation (see below) is possible can be obtained with CT-Angiography.
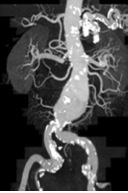
Figures:
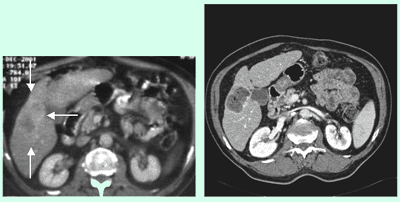
This is a CT Angiography of the abdominal aorta in an 85-yr-old patient. There is an aortic aneurysm. Because of the small distance between the aneurysm and the accessory renal arteries, it could not be treated by stent implantation.
Other common questions for which patients are referred for CT Angiography of the aorta are:
- Follow-up study after aortic aneurysm treatment
- Clarification or follow-up of an aortic dissection
- Search for the source of an embolus
Renal Arteries
In the setting of high blood pressure, the main question that CT Angiography must answer is whether or not one of the renal arteries is narrowed, i.e., a so-called renal artery stenosis.
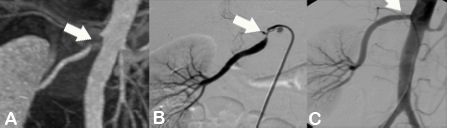
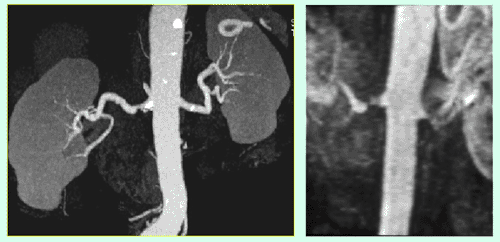
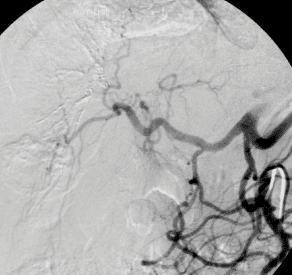
CT-Angiography, like MR-Angiography (see below) is an optimal noninvasive method to identify or exclude stenosis of either renal artery (see Fig.). A balloon dilatation with stent implantation, i.e., PTA and stent, (see below) is planned for this renal artery stenosis demonstrated on CT-Angiography. After successful stent implantation, a follow-up CT-Angiography was performed (gold standard).
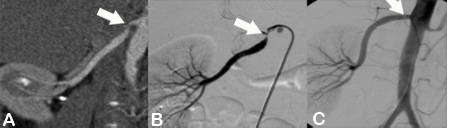
Figure A shows a CT-Angiography of the right renal artery in a young patient with poorly controlled high blood pressure. The CTA clearly shows a high-grade origin stenosis of the right renal artery (arrow). Based upon this CT-Angiography, the decision was made to do PTA and stent implantation.
Figure B shows the angiography immediately before stent implantation, confirmed by CT report (arrow).
Figure C shows the baseline angiography after stent implantation (arrow). The outcome was excellent.
Carotid Artery
The principle question at CT Angiography of the carotid artery is whether there is a relevant stenosis of the internal carotid artery (which supplies the brain) or the vertebral artery. The method of choice to address this question is a duplex ultrasound where the stenosis can be quantified. Based upon certain anatomical factors or existing calcification, in some patients duplex ultrasound is limited in its assessment. In such cases, CT-Angiography, like MR-Angiography (see below) is a very good alternative for carotid artery assessment.
CT Angiography, like MR Angiography (see below), is an optimal noninvasive method to diagnose or exclude a relevant stenosis of the carotid artery. On the basis of CT Angiography, balloon dilatation and stent implantation (see below) of a carotid stenosis can be planned and followed up.
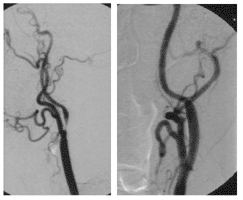
Figures A and B show a 67-yr-old man after stent implantation in the left internal carotid artery two years ago.
Fig. B shows the CT-Angiography of the left ICA stent. Its shape is maintained and it allows perfusion of the artery.
Fig. A shows a high-grade stenosis of the right ICA (arrow).
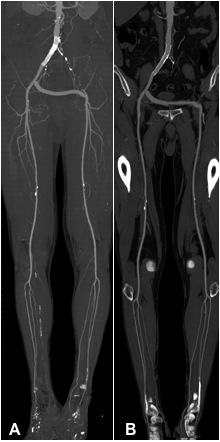
Pelvis and Lower Extremity Arteries
The main question that CT-Angiography of the pelvis and lower extremity arteries must address is whether there is intermittent claudication. CT-Angiography, like MR-Angiography (see below), is an optimal noninvasive method to diagnose or exclude narrowings in the pelvic and lower extremity arteries. Based upon the CT images, one can decide if surgery, e.g., bypass, or endovascular, i.e., balloon dilatation +/- stent implantation (see below) is preferable. Noninvasive follow-up CT-Angiography can be done after stent placement or bypass operation.
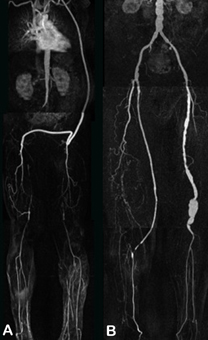
Figures A and B show a 74-yr-old man after stent implantation in the right pelvic arteries. There is a crossover from bypass from right to left for occlusion of the left pelvic arteries.
Fig. A shows a MIP-Depiction of the CT Angiography of the pelvis and lower extremity arteries. It is unclear if the stent is in the right pelvic arteries
Uniquely, our department has a MultiPath-CPR (Fig. B) that allows us to see inside the stent to determine if it is has restenosed.
Further areas where CT Angiography is performed include:
- Visceral arteries
- Subclavian artery
- Transplant arteries
Contraindications and Limitations
CT-Angiography has the same absolute and relative contraindications as computer tomography (CT) since you will be given intravenous iodinated contrast agent.The essential contraindications and preparation measures are summarized in Table 1. To determine any possible contrast agent contra-indications you may have, you will be asked to complete a questionnaire. The Information Form can be found at www.oerg.at
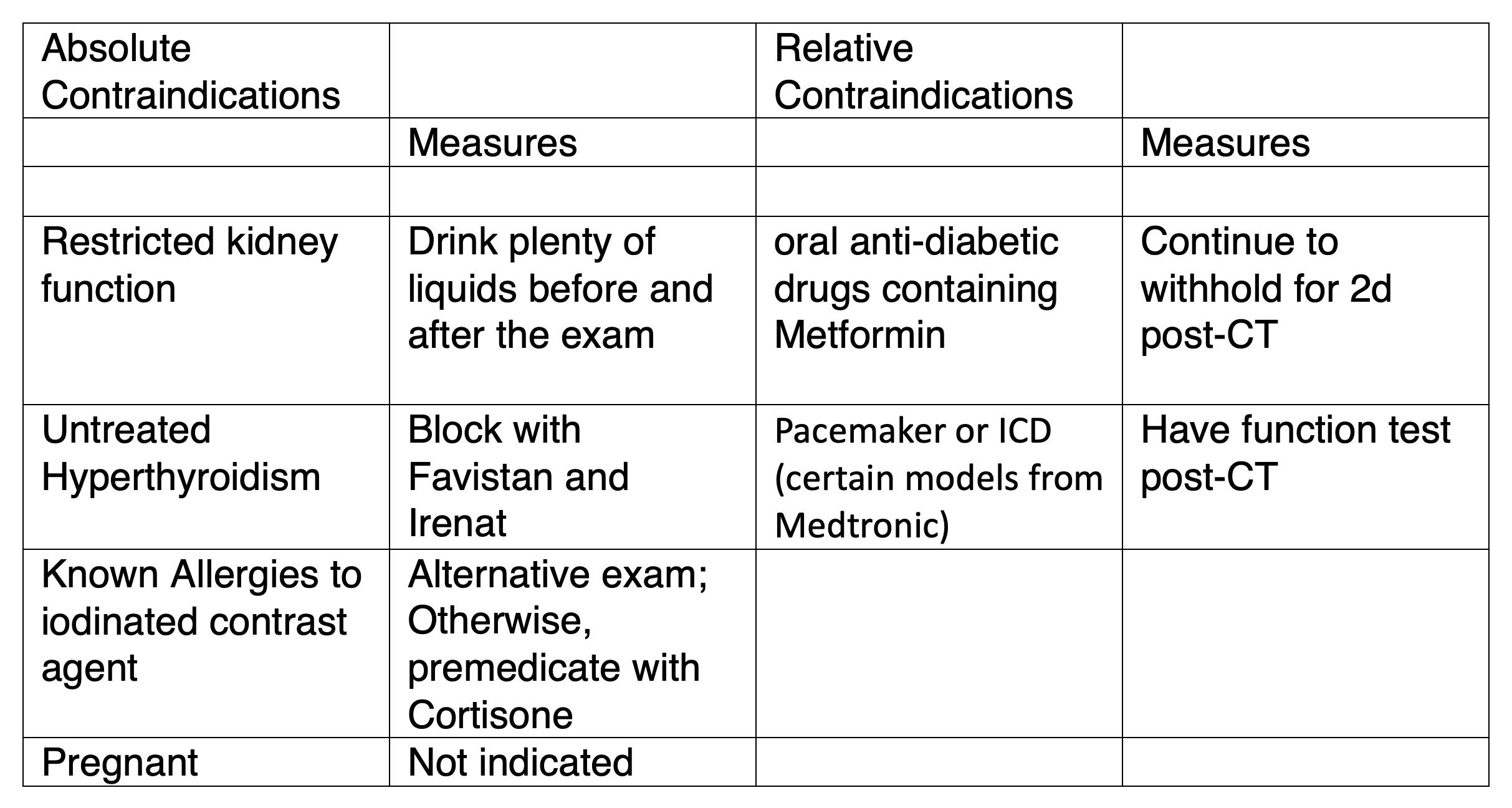
Table 1
General absolute and relative contraindications to CT contrast agents.
FAQ:
Can a CT-Angiography save me an arterial puncture, i.e., an angiography?
Many diseases of the arteries can’t be directly treated. Other arterial conditions are actually much less common than clinically suspected. For example, only 5 out of every 100 patients with high blood pressure will have renal artery stenosis. In such cases, noninvasive CT-Angiography can indeed save you an arterial puncture.
In other cases, after CT Angiography an invasive Angiography will be necessary for treatment, i.e., PTA, stent (see below). Optimal planning will be based upon CT-Angiography.
Doing a CT-Angiography before (or instead of) a planned Angiography is therefore in many cases meaningful and indicated.
When is a CT Angiography meaningful?
Almost all arterial conditions can be diagnosed or excluded by a CT-Angiography. The following list only gives a brief overview the most common indications
- Suspected or known aortic aneurysm
- Suspected renal artery stenosis
- Suspected carotid artery stenosis
- Intermittent claudication
- Abdominal angina
- Follow-up after all endovascular or surgical interventions of the arteries, e.g., bypass, balloon dilatation, PTA, etc.
Are there special contraindications to a CT-Angiography?
Theoretically, CT-Angiography is nothing more than a CT with contrast agent. The issue regards contraindications to the injected contrast agents, e.g., known contrast agent allergy, renal insufficiency, hyperthyroidism.(see above, also see the Information Sheet on the Austrian Radiography Society homepage, www.oerg.at)
Does one need special preparation for CT-Angiography?
No. As for any CT done with contrast agent, you must fast for 4 hours.
How long does a CT-Angiography last?
Including preparation, i.e., EKG placement when necessary, CTA lasts 10 minutes, maximum.
When should CT Angiography, when should MR-Angiography be performed?
It depends upon the individual’s contraindications: Patients with cardiac pacemakers, defibrillator implants, insulin pumps and claustrophobia, and patients after stent implantation will undergo CT-Angiography. Patients with renal insufficiency, diabetes, hyperthyroidism or iodine allergies shall undergo MR-Angiography.
The clinical question can decide whether CT or MR will be done. Generally, CT is the primary exam for queries about the aorta, while MR is preferred for queries regarding the pelvic and lower extremity arteries. However, the decision is sometimes made on a case-by-case basis.
MR-Angiography
Technical and contrast agent developments in the last 10 years have improved MR’s spatial resolution. Contrast agent will be injected intravenously at the MR exam to depict the arteries. This allows us to see most changes in the arteries noninvasively. This means a direct puncture in the artery, as with angiography (see there), is not necessary.
Theoretically, MR-Angiography (MRA) is equally useful to study vessels in any region of the body. It is slightly less useful if contrast injection is in the same territory being imaged.
Technique
Technical Requirements
Both spatial and temporal resolution have been improved with the availability of 1.5-3 T MRI. With modern receiver coils, the signal-to-noise relationship has also improved. Spatial resolution has also improved with the availability of high field strength magnets (i.e., 1.5 -3.0 T).
Course of the Exam
You will lie in the supine position for the exam.
Routinely venous access will be obtained for contrast agent administration, and you will be asked repeatedly to breathe in and then breath-hold.
MR-Angiography will be performed after injection of ca. 15–30 ml of Gadolinium-containing contrast agent.
Presently, these are the vascular studies our division offers:
Aorta
The principle question by MR-Angiography of the aorta (in the chest or abdomen) is whether the aorta is enlarged, i.e., a so-called aortic aneurysm. The urgency with which an aneurysm is treated will depend upon the diameter of the aorta. Treatment is warranted when an aortic aneurysm exceeds 6 cm in the chest or > 5–5.5 cm in the abdomen.
MR-Angiography, like CT-Angiography (see below) is an optimal way to decide if treatment is required. All parameters necessary to determine if endovascular treatment, i.e., stent implantation (see below) is possible can be obtained with MR-Angiography. However, due to the higher spatial resolution, CT-Angiography is the imaging method of choice. In patients with contraindications to CT-Angiography, MR-Angiography is the exam of choice.
Other common questions for which patients are referred for MR-Angiography of the aorta are:
- Follow-up study after aortic aneurysm treatment
- Clarification or follow-up of an aortic dissection
- Search for the source of an embolus
Renal Arteries
In the setting of high blood pressure, the main question that CT Angiography must answer is whether or not one of the renal arteries is narrowed, i.e., a so-called renal artery stenosis.
MR-Angiography, like CT-Angiography (see above) is an optimal noninvasive method to identify or exclude stenosis of either renal artery (see Fig). A balloon dilatation with stent implantation, PTA and stent (see below) is planned for this renal artery stenosis demonstrated on MR-Angiography. After successful stent implantation, a follow-up CT-Angiography is performed.
Figures:
Figure A shows an MR-Angiography of the pelvis and lower extremity in a patient with occlusion of the infrarenal aorta and both lower extremity arteries. This image shows that an aorto-femoral bypass and left to right crossover have been performed. Note that the thigh arteries are occluded on both sides.
Figure B shows an MR-Angiography of the pelvis and lower extremity in a patient who has had a right femoral-popliteal bypass. An aneurysm of the left popliteal artery is seen.
Further areas where MR-Angiography is performed include:
- Visceral arteries
- Subclavian artery
- Transplant arteries
- Arteriovenous Malformations
Contraindications and Limitations
MR-Angiography has the same absolute and relative contraindications as magnetic resonance imaging (MRI) with gadolinium-based contrast agent since this will be given intravenously. The essential contraindications and preparation measures are summarized in Table 1. To determine any possible contrast agent contraindications you may have, you will be asked to complete a questionnaire. The Information Form can be found at www.oerg.at
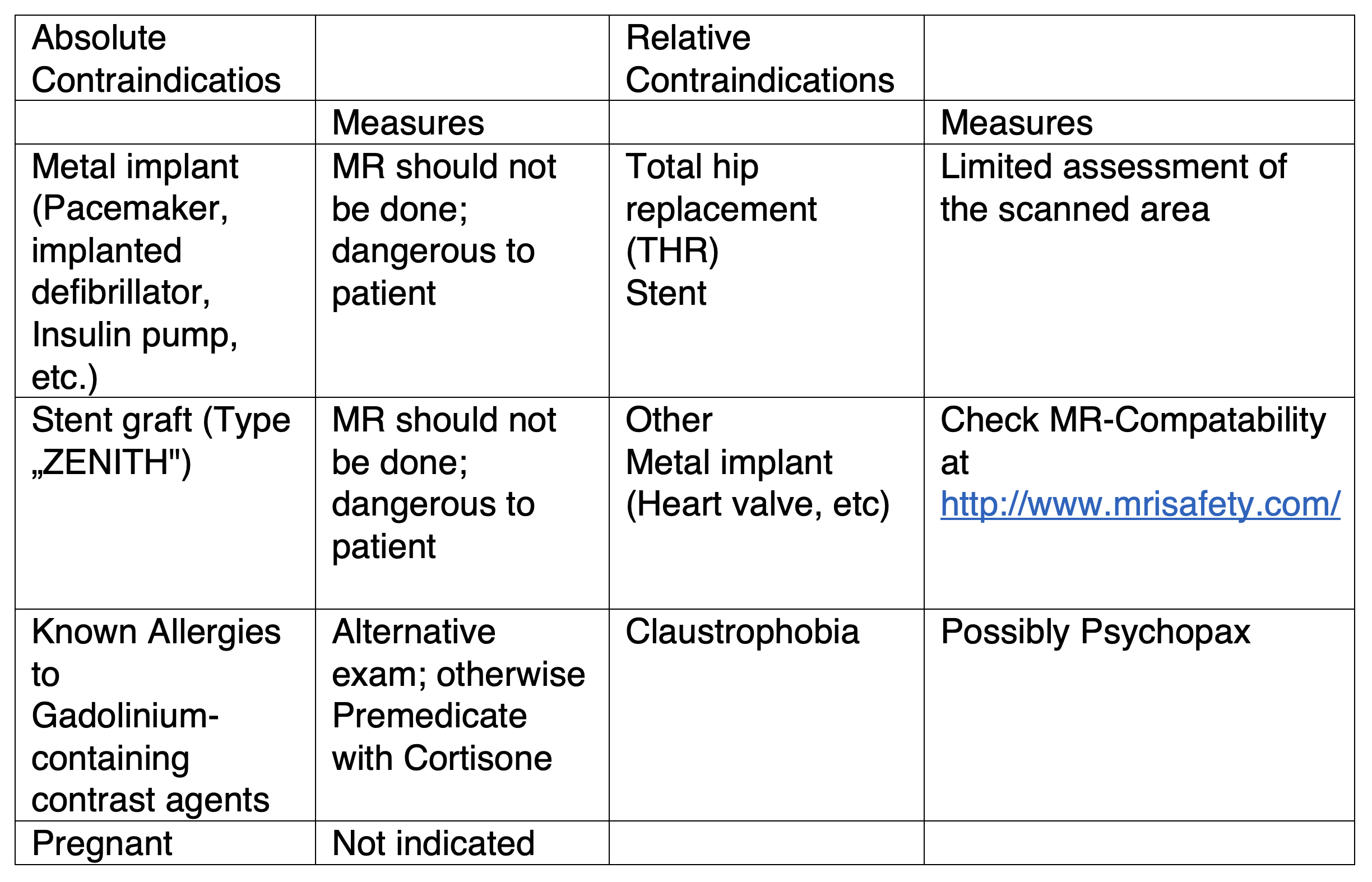
Table 1
General absolute and relative contraindications to MRI contrast agents.
FAQ:
Can an MR-Angiography save me an arterial puncture, i.e., an angiography?
Many diseases of the arteries can’t be directly treated. Other arterial conditions are actually much less common than clinically suspected. For example, only 5 out of every 100 patients with high blood pressure will have renal artery stenosis. In such cases, noninvasive MR-Angiography can indeed save you an arterial puncture.
In other cases, after MR-Angiography an invasive angiography will be necessary for treatment, i.e., PTA, Stent (see below). Optimal planning will be based upon MR-Angiography
Doing MR-Angiography before (or instead of) planned angiography is therefore in many cases meaningful and indicated.
When is a MR Angiography meaningful?
Almost all arterial conditions can be diagnosed or excluded by MR-Angiography. The following list only gives a brief overview the most common indications:
- Suspected or known aortic aneurysm
- Suspected renal artery stenosis
- Suspected carotid artery stenosis
- Intermittent claudication
- Abdominal angina
- Arteriovenous malformation
- Follow-up after all endovascular or surgical interventions of the arteries, e.g., bypass, balloon dilatation, PTA, etc.
Are there special contraindications to MR-Angiography?
Theoretically, MR-Angiography is nothing more than a MR with contrast agent. The issue regards contraindications to the injected contrast agents, e.g., known contrast agent allergy, pacemaker, etc. (see below, also see the Information Sheet on the Austrian Radiography Society homepage, www.oerg.at )
Does one need special preparation for MR-Angiography?
No. Also, you must not fast.
How long does an MR-Angiography last?
Including preparation, MRA lasts 30 minutes, maximum.
When should MR-Angiography, when should CT-Angiography be performed?
It depends upon the individual’s contraindications: Patients with cardiac pacemakers, defibrillator implants, insulin pumps and claustrophobia, and patients after stent implantation will undergo CT-Angiography. Patients with renal insufficiency, diabetes, hyperthyroidism or iodine allergies shall undergo MR-Angiography.
The clinical question can decide whether CT or MR will be done. Generally, CT is the primary exam for queries about the aorta, while MR is preferred for queries regarding the pelvic and lower extremity arteries. However, the decision is sometimes made on a case-by-case basis.
Renal PTA/Stent
Indications
High blood pressure can follow a narrowing in the renal arteries that reduces blood flow. In young patients who suddenly develop high blood pressure that is not relieved with appropriate medication, i.e., intractable high blood pressure, renal artery stenosis (RAS) must be considered. RAS should also be suspected if renal function worsens despite ACE inhibitors.
Primary Examinations to workup renal artery stenosis (RAS) are:
- Captopril Scintigraphy
- Computer Tomography Angiography (CTA)
- Magnetic Resonance Angiography (MRA)
- Color-Doppler Ultrasound
A combination of high blood pressure and worsening renal failure can be due to bilateral renal artery stenosis
Technique
The PTA/Stent treatment of RAS requires a 3 to 4-day hospital stay. On the day of the exam various bloodwork (blood count, blood clotting, renal function, blood lipids, blood glucose, etc.) will be compiled.
The groin will be numbed and then a balloon catheter painlessly placed in the renal artery. The balloon will dilate the narrowed part of the renal artery. If the artery does not widen sufficiently, a metal grill stent will be inserted into the artery. The catheter will be removed and a compression device applied to the groin wound.
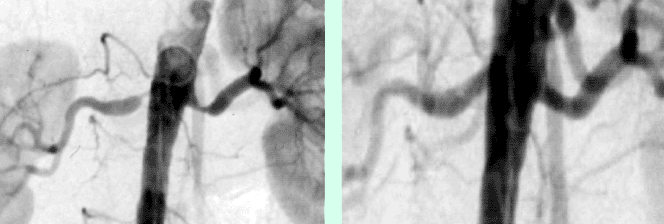
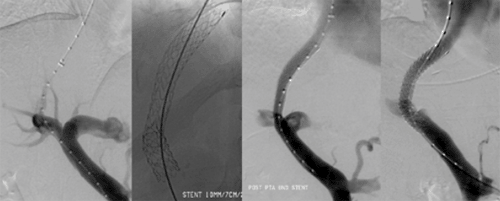
Figure 7a,b: Patient with high blood pressure (160/110 mmHg on medication). Bilateral renal artery stenosis before and after stenting.
Pre- and Post-treatment
During the PTA/Stent placement you will be given anticoagulation, i.e., Heparin 5000 IE. After successful PTA you will be given acetylsalicyclic acid (ThromboAss © 100mg/d po.) and LMWH (Lovenox© 2x40mg/d sc. for 2d). If you have had stent implantation, you will also be given Clopidogrel (Plavix© 75mg/d po. for 4 weeks, loading dose 4x75mg).
Your blood pressure medication must be adjusted.
Follow-up exams are recommended at 48 hours, 6 months and 12 months, either Duplex-US or MRA after PTA, or Duplex-US or CTA after stenting.
Complications
The worst complications would be renal artery rupture during PTA or vessel occlusion due to a dissection. Both occur seldomly (<1%). The most common complication is bleeding at the puncture site in the groin (~5%) with subsequent pseudoaneurysm. To avoid this, in high-risk patients we use a vascular closure device.
Other Therapies
First and foremost, you must decide whether the high blood pressure or reduced renal function can be treated with medication alone.
In young patient, balloon dilatation is always attempted. If the kidney function declines on medication, then PTA/Stenting will also be performed.
Nowadays, an aorto-renal bypass will only be performed if medication and PTA have failed.
Indications
A carotid artery stent is the first-line treatment for symptomatic stenosis. A narrowing of >70% in the carotid artery and neurologic symptoms (arm or leg weakness, hemihypoesthesia, i.e., sensory loss on one side of the body, visual disturbance) within the last 6 months.
Prophylactic treatment for an asymptomatic patient with a stenosis >70% is optional. Frank occlusion of the carotid can‘t be treated.
Technique
After local anesthetic, the groin artery will be puncture. A thin catheter (ca. 2,0–2,5mm) will be advanced into the carotid artery using fluoroscopic guidance. The narrowed area, i.e., stenosis, will be probed with a thin guidewire. Then the metal mesh stent passed over the wire and inserted in the narrowed area. Placing a 5–6mm balloon within the stent, the vessel will be dilated. All catheters will be removed and the groin puncture site closed.
Pre- and Post-treatment
You will be admitted to the hospital the day before your scheduled stent placement. The carotid will be imaged with ultrasound and an MRI done to view the brain. Blood will be drawn to check your blood count, clotting, renal function, blood glucose, and lipids. You will be started on anticoagulants (i.e., blood thinners), including Clopidogrel (Plavix© 4x75mg), acetylsalicyclic acid (ThromboAss© 100mg), and Heparin (Lovenox© 2x40mg).
After the procedure you must be on bedrest for 24 hours. An ultrasound of the neck will be done the following day. An MRA will be done 1-2 days later to assess the blood flow through the brain. You will be give additional medication, i.e., Plavix© 75mg/d (for 4 weeks), ThromboAss© 100mg/d, and Lovenox© 2x40mg/d sc. for 2 days.
Complications
The risk of developing an embolus from the carotid, advancing into the brain circulation and causing subsequent neurologic deficits, is 6% if you had a symptomatic stenosis pre-stenting. The risk falls to < 3% if you were asymptomatic before stenting. Introducing a filter system into the artery helps to minimize chances of this complication.
Seldom, patients develop hyperperfusion syndrome due to the sudden increase in brain blood flow following stent placement. This can cause hemorrhage into the brain.
In <5% of cases, bleeding will occur at the groin puncture site.
Other Therapies
Carotid artery stenosis increases the risk of stroke. For prophylaxis, starting anticoagulants, i.e., blood thinners, such as ThromboAss© or Plavix© makes sense. Surgical treatment is called carotid endarterectomy (CEA) and usually requires anesthesia. It entails cutting open the artery and shelling out the excess tissue. Complications include stroke, < 6%, and nerve damage, usually temporary, <5%.
Interventional Oncology
Definition:
In summary, interventional oncology refers to the procedures involved in treating malignant tumors. This includes both follow-up imaging of the tumors (with ultrasound, CT, MRI or fluoroscopy), and minimally invasive treatment (i.e., either accessing the tumor through a thin needle in the skin or through a catheter placed in a blood vessel).
In most cases full anesthesia is not necessary. A light sedative is adequate.
Due to new substances and technical developments in the last years, today minimally invasive treatment of tumors is on equal footing with surgery, chemotherapy and radiotherapy.
In many instances, interventional oncology procedures are performed in addition to conventional surgical or medical (i.e., chemo- and radiotherapy) therapy to strengthen the treatment-effect. Further, sometimes interventional oncological treatment is used exclusively, e.g., when surgery or chemotherapy is not possible or the tumor is unresponsive,
The following is an introduction to the most important interventional oncology procedures offered by our service.
1. Chemoembolisztion
During chemoembolization a catheter is introduced into an artery and then advanced into the branch artery that supplies the tumor. By injecting a mixture of chemotherapeutic particles and oil drops usually, the feeding artery to the tumor is closed off. This prevents blood and oxygen from reaching the tumor, and also delivers a higher dose of chemotherapy to the tumor than with conventional venous infusion of chemotherapy.
Another method of chemoembolization uses small plastic particles, i.e., average diameter 0.3 to 0.7 mm, coated with chemotherapy agent. These seal off the small feeding arteries to the tumor, again increasing the chemotherapy dose delivered to the tumor. The Division of Cardiovascular and Interventional Radiology led the European-wide study that proved the effectiveness of this particle.
Chemoembolization is especially used for treating tumors or metastases in the liver. Because the liver has two independent blood supplies (i.e., the hepatic artery and portal vein), sealing off the feeding artery to the tumor does less damage to healthy liver tissue.
In certain circumstances, e.g., painful bony metastases, chemoembolization is also used to treat malignant lesions in the skeleton.
2. Radioembolization
In the past, radioembolization also relied on the injection of radioactive-coated particles into the feeding artery of a tumor. Since the range of radioactivity from these particles was very small, they could be left in place for a few days while they decayed.
These particles are made from plastic or glass and contain Yttrium-90.
They were specially developed for treatment of aggressive tumors or liver metastases.
Since 2007, our division along with the Division of Nuclear Medicine have been offering this treatment. These particles can deliver an extremely high radiation dose to the tumor, with minimal radiation dose to the rest of the body.
This exam requires extensive preparation to ensure that particles are injected only into the feeding artery of the tumor. Particles elsewhere could cause damage to other organs, such as the pancreas, lungs or stomach.
Radioembolization can be used for treating liver tumors when the disease is exclusively or almost exclusively limited to the liver.
3. Thermoablation
Briefly, in thermoablation a needle-shaped electric antenna is inserted into a tumor (either in liver, lung, kidney, bones or other organ) via the skin. Cells within a 5–7 cm area die when exposed to the heat from this device.
Depending upon the procedure, either radiofrequency, microwaves or a very strong electric current is applied to the tumor area.
Thermoablation can be used anywhere, as long as the tumor is less than 5–7 cm, there are a limited number of separate tumors within the lesion, and no heat-sensitive organ lies near the tumor, e.g., bowel, heart biliary tract. In the peripheral areas on the lungs, liver kidneys or other organs, thermoablation can be safely used as the risk of complications is minimal.
Thermoablation is often performed in addition to surgery, e.g., when a large liver tumor is resected but smaller tumors in other segments are left behind. Also, in certain cases, thermoablation can be used to treat residual tumor.
Interdisciplinary Conferences
The successful use of interventional oncology techniques requires a close working relationship with various specialists. The Division of Cardiovascular and Interventional Radiology holds a weekly conference with surgeons, oncologists, radiation therapists and organ-related specialists to discuss treatment options and optimize treatment planning.
Liver Tumor Embolization
Tumor embolization
Liver tumors often have a rich blood supply which has developed as they grow. The goal of tumor embolization is to cut off this blood supply, preventing further growth. This will lead to tumor necrosis, i.e., it will either die or become smaller. Embolization can’t remove the tumor; it can only damage it, at best, or stop its growth, in the worst case scenario.
Liver tumor and Embolization
Not all liver tumors are suitable for this type of treatment. Small tumors can be resected, and, depending upon the location and size, they can also be ablated with ethanol, radiofrequency or laser. Tumor embolization is also used preliminarily in all tumors that have a large blood supply.
Preparation (Exams)
Before tumor embolization, CT is done to determine the exact dimensions and location of the tumor. Then you can decide which therapy is most suitable for the tumor. Additionally, the liver function and the entire blood supply to the tumor must be determined. Basically, the liver has two blood supplies, i.e., the arterial and portal venous systems. Although the arterial system supplies the majority of tumors, the portal venous system is largely responsible for supplying healthy liver tissue. Therefore, it is important to leave enough residual blood supply for healthy liver tissue to function, i.e., secure the portal venous supply. This can be checked with CT, too.
Technique
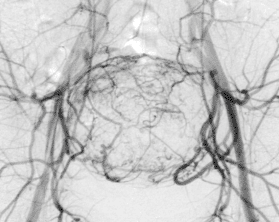
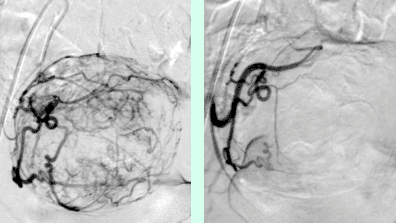
The job of the Division of Cardiovascular and Interventional Radiology is to determine if the tumor is suitable for embolization, and to secure liver function. A small puncture is made in the right groin to introduce the catheter that will be advanced into the liver tumor. The fine-tuning of the catheter within the tumor is very important. We use a microcatheter, i.e., super selective placement in the tiny arterial feeders to the tumor, for this. From this launching pad, 300–900μ particles are released into the feeding arteries until they fully block the vessel lumen. Then a bit of Glubran, i.e., a soft tissue glue, is put into the artery to ensure that it is sealed off. Using angiography, we can see where and how well we have launched the particles, and also if there are additional feeding arteries to the tumor.
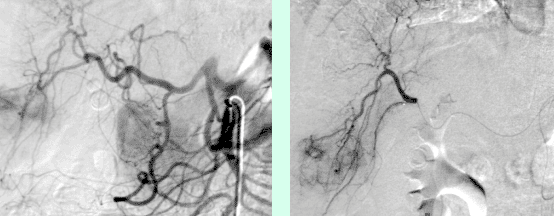
Figure 1: Overview angiography of the liver and superselective position of the microcatheter before embolization.
The groin puncture follows local anaesthesia. During the procedure, you could experience nausea and pain. Therefore, you will be given a sedative and also prophylactic antibiotics via intravenous fluids.
When we block off the artery feeding the tumor, it is unavoidable that we also cut off blood supply to some healthy tissue, too. The damage to this tissue will reduce your liver function somewhat. Embolization is usually done in 2 or 3 stages to allow any damaged liver tissue time to heal and regrow in between sessions. In planning, we take into consideration the entire situation of the patient.
Post-embolization Syndrome
From hours up to 2 days after the procedure, post-embolization syndrome, including nausea, fever, and eventually pain, can occur. Taking appropriate measure beforehand, and also through use of medication, we have minimized these unpleasant after-effects.
You will remain hospitalized for a few days after the embolization, before being discharged home. A follow-up CT will be done 5-6 weeks later to judge the success of the treatment.
Literature
Loewe C, Cejna M, Schoder M, Thurnher MM, Lammer J, Thurnher SA.
Arterial embolization of unresectable hepatocellular carcinoma with use of cyanoacrylate and lipiodol. J Vasc Interv Radiol. 2002 Jan;13(1):61-9
Radiofrequency Ablation
General Information
Radiofrequency ablation (RFA) is a minimally-invasive treatment method which uses high-frequency electrical current in a circumscribed area to produce high temperatures (up to 100° C), thereby destroying tissue. CT- MRI- or ultrasound-guidance is used to place a special needle into the tumor. The needle will be left in the tumor for ca. 15-20 minutes, depending upon the size of the tumor. This will destroy the tumor and a small “safety zone” around it.
With which tumors can Radiofrequency ablation be used?
Liver cell cancer (i.e., HCC-hepatocellular Carcinoma): The primary treatment of HCC is surgical resection or transplantation. RFA can be used for tumors ≤ 5 cm and when no more than 5 tumors maximum are present within the liver.
Liver metastases from colon cancer: in cases where surgery is not possible or is unwise, RFA may be used, as long as the lesions are ≤ 5 cm, and do not number more than 5.
Radiofrequency ablation can also be used in lung and kidney tumors. Fewer cases have been done and evaluated.
Radiofrequency ablation can be used as a palliative measure for bone tumors and painful compression fractures. RFA reduces the tumor burden, helping relieve pain.
Radiofrequency ablation has replaced surgery as the primary treatment for the benign bone tumor, i.e., Osteoid Osteoma
How is Radiofrequency ablation performed?
If the interdisciplinary team of physicians (i.e., surgeons, internists, orthopaedists or urologists) decides that RFA is appropriate for your case, then you will be admitted to the hospital. The procedure will be done by a radiologist in the Division for Interventional Radiology. Depending upon tumor location and size, you will be given either general anesthesia or sedation, i.e., an injection of strong pain and sleep medication to put you in a coma-like state. Using CT-, MRI- or ultrasound-guidance, the radiologist will place a needle into the tumor, via the skin. The needle will sit within the tumor for ca. 15–20 minutes, depending upon the size and location of the tumor. The entire procedure lasts ca. 60–90 minutes. Usually you will be hospitalized for 1-2 days for pain control and to make sure no complications occur.
What are the potential complications?
Radiofrequency ablation is a secure and proven technique. Complications can occur that make it necessary for you to go to surgery, e-g-, bleeding, injury to an adjacent organ. The exact risks are stated on the Information Sheet.
What is the advantage of Radiofrequency ablation?
Compared to surgery, radiofrequency ablation is gentler on your body (i.e., minmally-invasive procedure) and has minimal morbidity and mortality. However, risk of complications depends upon your general state of health, as well as the size and location of the tumor.
How do you contact us?
For us to determine if RFA is suitable for you, we will need the following:
- a detailed medical history from your doctor
- all of your pertinent imaging exams
- a letter of referral
This you can personally bring along or you can mail it to the following address:
Universitätsklinik für Radiologie und Nuklearmedizin
Klinische Abteilung für Kardiovaskuläre und Interventionelle Radiologie
Medizinische Universität Wien/AKH Wien
Leitstelle 8F
Währinger Gürtel 18-20
1090-Wien
Tel.: +43 (0)1 40400-58020
Fax: +43 (0)1 40400-58300
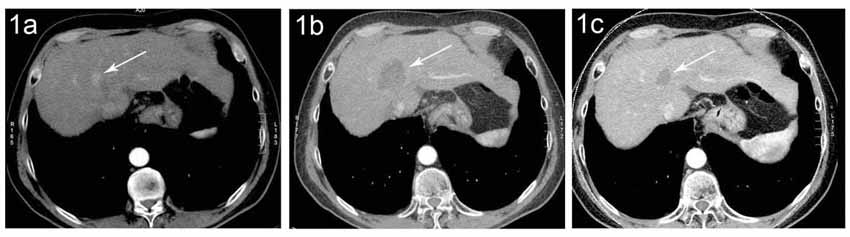
Figure (1a) CT of a small HCC (arrow) in a patient with liver cirrhosis (1b) Six months after RFA there is a necrotic area (arrow) without evidence of residual tumor in the area of former HCC. (1c) Four years later, the necrotic area is clearly smaller. Tumor recurrence can‘t be excluded.
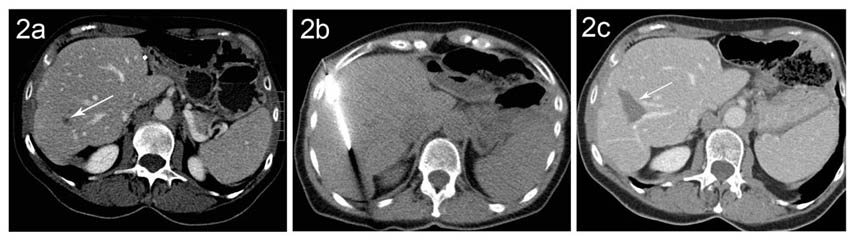
Figure (2a) CT from a patient with a solitary small recurrent colorectal cancer liver metastasis in the right liver lobe (arrow). (2b) CT-guided placement of RFA needle with the tip in the metastasis (2c). On the follow-up exam, a necrotic area is seen (arrow) where the metastasis had been No evidence for residual tumor noted.
TIPS (Transjugular Intrahepatic Portosystemic Shunt) for treatment of portal hypertension
Liver Cirrhosis
Liver cirrhosis, denoted by shrinkage of the liver, results from various different etiologies. In the industrialized world, alcohol is the leading cause of cirrhosis, followed by viral hepatitis (types B and C), metabolic diseases and rarely autoimmune diseases.
The dying liver tissue is replaced by connective tissue, the liver develops scars, both leading to liver cirrhosis. During this remodeling, the blood vessels within the liver also become narrower. It can no longer transport the necessary blood to the heart. The inflowing blood stagnates, backing up into the portal vein and raising the pressure there. This is called portal hypertension.
Complications from this include ascites, i.e., fluid in the belly, bleeding varicose veins of the esophagus, and hepatic encephalopathy.
TIPS
A transjugular intrahepatic portosystemic shunt (TIPS) is a method of relieving the high pressure in the portal vein by making a bypass circuit. The part of the liver threatened by narrowed vessels is joined to the area with stagnant (accumulating) blood. In some cases, the TIPS is only a transitional solution, buying time until a necessary liver transplantation is performed.
Technique
For the procedure, you will be given pain medication and sedatives, as needed, to reduce your discomfort. Under certain circumstances, general anaesthesia is possible. Then a catheter will be placed in a vein on the right side of the neck and advanced into the liver. This catheter will be used to transport all necessary devices for the TIPS into the liver. Using a needle, a puncture will be made to connect the right hepatic vein to the portal vein. Using a guidewire and balloon, this new passage, i.e., a bypass circuit, will be widened and then a metal stent placed in the bypass to hold it open so that blood can flow through it.The pressure of the portal vein will be measured. The goal of the bypass is to bring enough blood through it to relieve the stagnant blood in the rest of the liver, but still leave enough blood in the congested liver so that it can remove toxins.
Once a TIPS is in place, over time, the blood flow through the bypass may decrease again. Therefore, a repeat TIPS will have to be done to enlarge the bypass circuit. Otherwise, blood will back up into the varicose veins surrounding the esophagus, and possibly bleed. The narrowing of the stent naturally occurs as tissue grows in or on the periphery of the metal stent. This is why angiography must be done periodically, i.e., to check the blood flow through the stent. When blood flow becomes too little, the stent will be dilated.
Recently, our division has gotten a new type of stent that is almost completely made out of Goretex (i.e., PTFE)This plastic membrane prevent tissue buildup inside the stent, thus preventing narrowing of the stent.
Adverse Effects
One of the most important unwanted effects is new-onset or worsening hepatic encephalopathy This is a decrease in brain function due to blood toxins, i.e., ammonia, which is built up due to a protein-rich food. Typically, the ammonia would have been removed from circulation by normally-functioning liver. But the TIPS, i.e., bypass, means that a substantial part of circulating blood no longer passes through the liver. As the toxins in the blood increase, the signs of hepatic encephalopathy, i.e., disturbances in motion, concentration and ability to reason, increase.
Indications
- Acute variceal bleeding: if through endoscopic and medical (i.e., Terlipressin, Somatostatin) intervention the bleeding can’t be controlled
- Prophylaxis of esophageal variceal bleeding: Standard therapy includes ß-Blocker (e.g., Propranolol) und Nitrate. The prophylactic use of TIPS is not yet proven.
- Secondary prophylaxis of esophageal variceal bleeding (after initial bleeding): TIPS is practically as good as endoscopic therapy, i.e., sclerosis, ligation. (Rössle M. et al Lancet 1997)
- Treatment-refractory Ascites: proven use in cases in which ascites quickly returns after paracentesis (Ochs A. et al. NEJM 1995; 332: 1192-1197). If liver function worsens, a Denver Shunt (i.e., peritoneal-venous shunt) should be discussed as an alternative.
- Budd-Chiari-Syndrome: A TIPS can effectively treat the liver and bowel congestion.
- Portal vein thrombosis: Acute portal vein thrombosis can be treated effectively by local lysis or eventually by TIPS. A prerequisite is that the inflowing mesentery veins will have sufficient blood flow after the TIPS.
- Pressure volume due to excess splenomegaly: This is an uncommon indication that arises in patients in whom a splenectomy is not advised. Most have good liver function so that one does not have to deal with liver insufficiency, should there be any TIPS complications.
- The following considerations must be taken into account regarding TIPS if endoscopic treatment of bleeding esophageal varices fails:
- Judge the risk of recurrent bleed without TIPS (based upon endoscopy, i.e., if have Grade 3 and 4 varices, red spots, gastric fundus varices)
- Judge the risk of bleeding complications, such as encephalopathy, liver insufficiency, and kidney insufficiency (based upon the Child-Pugh-classification)
- Judge the risk of hepatc encephalopathy after TIPS (based upon the Child-Pugh-classification)
- Judge the risk of liver insufficiency during TIPS by icterus and decrease in coagulation values (based upon the Child-Pugh-classification and experience with previous episodes
- If by a high risk of bleeding that causes overall clinical worsening, the need for prophylaxis is very high, but the risk of complications after TIPS is increased. Nonetheless, such patients may benefit from a TIPS.
- Bleeding patients with elevated ammonia levels probably benefit from endoscopic therapy (ligation, sclerosis), and also from TIPS if they have ascites too. (Rössle, M. et al. Lancet 1997).
Contraindications
Relative Contraindications
- Hepatic encephalopathy
- Liver insufficiency (< 12 P Child-Pugh)
- Acute infection
Absolute Contraindications
- Polycystic liver disease
- Portal vein thrombosis with cavernous transformation
- Hepatopulmonary Hypertension
- Heart insufficiency
- Liver tumor
- Liver failure (<12 P Child-Pugh)
Peripheral artery disease (PAD)
The incidence of peripheral artery disease (PAD) and also intermittent claudication increases with age, affecting ca. 20–30% of the population eventually.
Only one-quarter to one-third of those with complaints come to medical attention. Various causes exist for PAD, the most frequent being atherosclerosis (95%).
The main risk factors for atherosclerosis in the lower extremities are cigarette smoking, diabetes, high blood pressure and hyperlipidemia.
The severity of peripheral artery disease (PAD) is divided into four grades.
Grade I – asymptomatic PAD
Grade II – Intermittent claudication: can walk > 200m (II A)
can walk < 200m (II B)
Grade III – Pain at rest
Grade IV - Necrosis, Gangrene
The first-line treatment of PAD addresses the underlying causes, i.e., smoking cessation, controlling high blood pressure, treating diabetes, and lowering blood lipid levels
Starting from Grade II B (i.e., ability to walk < 200m), conservative measures are no longer a treatment option. Revascularization is necessary, potentially using interventional radiology or vascular surgery.
The typical interventional radiology procedure is the percutaneous transluminal angioplasty (PTA) in which narrowed or occluded arteries are dilated with balloon catheters. The access to the diseased artery is through the groin where various catheters and wires are introduced.
In certain cases, i.e., when dilatation is inadequate or when the vessel is dissected, a stent will be placed in the vessel lumen, over a guidewire, to keep the vessel open.
If the root causes of PAD symptoms can’t be treated, e.g., through smoking cessation, other medications will be used.
Every procedure comes with risks. The most important risks and complication of PTA are:
- Bleeding and/or infection (at the puncture site)
- Vessel dissection (a split in the vessel wall)
- Thromboemboli and occlusion
- Vessel rupture
- Unsuccessful or inability to carry out the procedure
In most cases, these complications can be repaired during the same session or in a subsequent interventional radiology session or reversed through conservative measures. In ca. 1–2%, surgery will be necessary to repair the vessel. The risk of a fatal complication is clearly < 1%.
All in all, interventional radiology therapy for peripheral artery disease (PAD) has relatively few risks and complications.
After a successful intervention you must stay on bedrest for 24 hours to allow the puncture site to close. Increasingly, due to modern vessel closure systems, 20, rather than 24 hours of bedrest is necessary.
For the natural course of PAD, as well as the long-term success of PTA, it is necessary to reduce your risk factors, i.e., cigarette smoking, diabetes, high blood pressure, etc.
Embolization
Technique
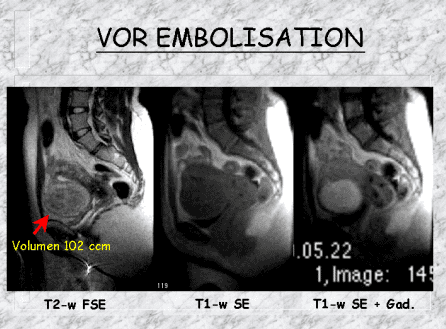
Embolization is a well-known medical technique that has been used since the 1960s to treat bleeding and tumors all over the body. It has also been used to treat uncontrolled uterine bleeding post-partum or in tumors. Since the early 1990s, a Parisian team developed a method to embolize uterine myomas. Since 1995, this technique has increasingly been used. Worldwide, more than 10,000 embolizations have been performed.
After local anesthetic, a thin plastic tube will be introduced into a blood vessel (i.e., the femoral artery) in the groin and, using contrast agent and radiography, will be advanced into the uterine artery branch that feeds the myoma.
Particles (0,3–0,7 mm) will be injected into the myoma via the catheter and branch out into the tumor, blocking off its blood supply, i.e., embolization. Using radiography, when the bleeding has stopped, the catheter will be moved into the artery on the opposite side of the uterus and the same process repeated. It is necessary to stop the blood flow to the tumor from both sides. Otherwise, the blood flow to the still-patent artery will increase to compensate for the blood flow lost from the opposite artery. As no blood feeds the myoma, it will gradually involute.
Finally, the catheter will be removed from the groin and pressure applied to the puncture site with the fingers. For safety, a compression device will be applied to the groin and you will have to stay on bedrest for 24 hours. Eating and drinking is allowed immediately after the procedure.
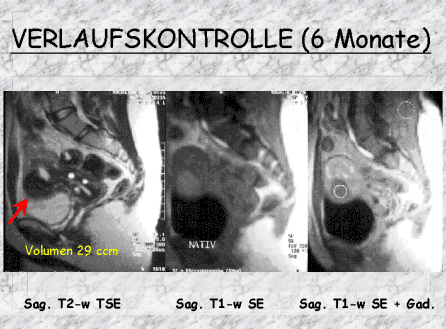
In more than 90% of patients successfully treated, during the following months, the myoma will shrink by 25-50% (ca. 60% within 6 months, ca. 70% within 12 months). The blood supply to the healthy uterus only will be taken over by other vessels.
Pain therapy
Now that the main blood supply to the uterus has been interrupted, the uterus swells. Without medication, this is painful and can cause nausea and fever, i.e., the so-called post-embolization syndrome.
To prevent this scenario, the patient is given a PCA (i.e., patient-controlled analgesia) pump through which pain medication is continuously delivered intravenously. But the pump also allows the patient to temporarily increase the amount of pain medication given should an intense bout of pain occur.
After a day, the pain will be so mild that it will be controlled with suppositories rather than the pump. The suppositories are usually necessary from 7 to 10 days.
To prevent infection, a dose of intravenous antibiotics is given before the embolization. Additionally, if the myoma is large, antibiotics in tablet form are given for one week.
Success rate
Technical success is achieved in 98% of cases. Pain is successfully treated in over 90% of cases.
Potential complications
Post-embolization syndrome: The above-mentioned complaints occur in most cases, proving the successful tumor death after the embolization. These symptoms are temporary.
Infection: Infections complicate ca. 1–2% of cases. The dearth of blood flow to the embolized tissue predisposes to infection. To prevent this, the patient is given a dose of antibiotics. If an infection occurs, additional antibiotics are given. Rarely, pyometra (i.e., pus in the uterus) occurs,requiring hysterectomy. The risk of infection increases with the increasing size of the myoma.
If the patient does not consent to a hysterectomy under any circumstances, the embolization will not be done, despite the very low risk that this would happen.
Evidence of a possible infection is purulent liquid coming from the vagina, increasing and sharper pain in the lower abdomen, and onset of fever. In any such case, the covering staff doctor or gynecologist shall be consulted to determine treatment.
Infections can also occur distant from the procedure, e.g., 3 months after the embolization.
Embolization particle diversion: Because the arteries to the bladder and vagina are near the uterine artery branch, theoretically the plastic particles can wander into these branches, causing mucosal membrane damage. This really occurs very rarely.
Menstruation failure: In most cases, the menstrual cycle remains intact after myoma embolization, and the blood flow will be decreased after successful embolization.
In a few cases, menstruation may be absent temporarily. But it usually normalizes without intervention.
In exceptional cases, i.e., 1%, menstuation prematurely disappears permanently. This is usually in patients who are near menopause.
Expulsion of myomas: In ca. 10% of cases – especially when the myoma is located directly beneath the uterine cavity mucosa—the myoma can be ejected from the vagina. This usually happens within 3 months of the embolization and can be preceded by periodic pain and bloody discharge.
This isn’t a complication, though it is unpleasant. However, if the desquamated tissue remains in the uterus, it can cause an infection. If the patient has the sensation that she has retained desquamated tissue, she should consult a physician
Other complications: Other complications of the procedure are related to the catheter, contrast agent, local anesthetic, bleeding, vessel injury or vessel occlusion. These complications occur seldom. Bleeding from the puncture site is more common, but usually inconsequential.
Post-embolization care
For up to 2 weeks after the procedure, the patient can feel worn out, tired. Therefore, plan to be away from work for two weeks. A discharge may occur that will slow within 3 to 4 days, but can sometimes last for as long as 2 weeks. Mild lower abdominal pain—similar to menstrual pain—should be expected during the first week. You can take anti-inflammatory medications. Watch for any signs of infection (see above).
The patient should see her gynecologist for further care soon after discharge from the hospital.
Since the uterus has been left in place, you can plan to become pregnant. However, you should have a curettage first. Following the operation, it’s possible to get concretions in the peritoneal cavity that block the fallopian tubes or cause a tubal pregnancy. If heavy bleeding occurs during the operation, a hysterectomy may be necessary.
Vertebroplasty
Vertebroplasty refers to a simple procedure of pushing cement into the fractured vertebral body to try to relieve the pain. The procedure has existed for several years and has been performed in thousands of patients already. Through the development of instruments and materials, this minimally-invasive technique has advanced so that it can be performed in less than an hour with a high success rate and less than a day’s hospitalization.
The Vertebral Body
The lumbar vertebra are the most common cause of painful fracture, despite their relatively large size. The vertebra is composed of the body and two pedicles which project posteriorly to form a closed canal. Between any two adjacent vertebral bodies, at the level of the pedicles, the nerves exit from the neural foramina. This plays a key role why a vertebral body fracture may cause narrowing of the neural foramina.
The spinal canal lies within the bony ring formed by the vertebral body and pedicles.
In the lumbar spine only, the nerve fiber branches run. In the cervical and thoracic spine, the spinal cord runs.
Vertebral body compression factures have various causes. osteoporosis fractures, due to loss of calcium, weaken the bone. These fractures usually occur in post-menopausal women, but can also occur from trauma, a fall or tumor.
Preparation for the procedure
Basic to the treatment is the correct diagnosis. This is usually done by physical exam and then radiography. If treatment is being planned, then computer tomography (CT) must also be done. Which vertebral body should be treated can only be decided by the expert, i.e., specialized physician. Indeed, the success of the treatment is based upon this selection. Once the Division of Cardiovascular and Interventional Radiology is given the indication for the exam, we perform the vertebroplasty.
Once the patient, expert (i.e.,specialist) and interventional radiologist agree on the level of the vertebroplasty, an appointment is scheduled. Once the patient receives a so-called “internal release” that approves the procedure from the clinical side, then his blood counts, including his coagulation status are done. On the day before the procedure, the procedure will be explained to the patient in detail. The patient must take nothing by mouth (i.e., fast) on the day of the intervention, except for his prescribed medications.
Technique
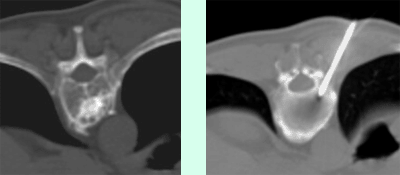
The intervention is performed under local anaesthesia and under sterile conditions. The suite resembles a small operating room, with an operating table and a CT which guides the radiologist during the procedure. The patient lies on his belly (i.e., prone). The intervention occurs from his backside.
The size of the puncture is related to the size of the needle. A monitor that can continuously be viewed shows the shortest and safest means of placing the cannula into the vertebra. Once the cannula is in the desired vertebral body, the sterile bone cement can be injected under visual monitoring. Once the cement is in place, the cannula is removed. The procedure is finished, but you must remain lying in bed. You will be transported to your room.
Safety
The large machines and sterile operating room can make some patients nervous, insecure. Both CT and fluoroscopy (i.e., double security) are used to insure the precision of the procedure, i.e., that the cannula slides by the nerve to achieve a stable position within the vertebral body. Only by guiding the needle under image-guidance can we avoid any error. Remember that behind all this equipment, are the people that you have already spoken with before the exam and whom you can speak to during the procedure. We will react immediately to any complaints you may have.
Post-vertebroplasty care
After a short time lying on the stretcher, you will be able to stand up, and also eat or drink. .In uncomplicated cases, you will be able to go home that same evening, after a checkup by your specialist. Typically, a follow-up CT is done 6 months after the vertebroplasty. Early imaging is only done if you develop pain or other symptoms.
Adresses
Special clinics in Austria that also offer vertebroplasty: KH der Elisabethinen, Linz, Abteilung für Radiologie OA Dr. Gschwaendtner
AKH Wien: Klinische Abteilung für Kardiovaskuläre und Interventionelle Radiologie